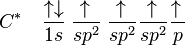
The simplest of the organic molecules known as alkenes, which contain carbon-carbon double bonds, is ethylene (CH2=CH2). It’s a combustible, colourless gas with a sweet flavour and odour. Both natural gas and petroleum are natural sources of ethylene; It’s also a hormone found in plants that slows growth and encourages leaf fall, as well as in fruits that stimulate ripening.
Ethylene is a widely used organic molecule in industry. It is made by heating either natural gas or petroleum to 800–900 °C (1,470–1,650 °F), resulting in a mixture of gases from which the ethylene is extracted. Ethylene has a melting point of 169.4 °C [272.9 °F] and a boiling point of 103.9 °C [155.0 °F].
Ethene was not mutagenic to one strain of Salmonella typhimurium with and without the liver metabolic activation system at ambient concentrations up to 20%. (S9). Ethene was negative in other Salmonella strains in the presence and absence of S9.
Physical properties
At room temperature and pressure, a colourless gas exists
-169°C melting point
-104°C is the boiling point.
The scent is slightly pleasant
flammable
Non-polar molecule that is soluble in non-polar solvents but not polar solvents such as water
The double bond is the active site in this reaction
Ethene, for example, is a good candidate for additional reactions
Chemical Properties of Ethene
Addition of Chlorine:
CH2=CH2+Cl2CH2Cl-CH2Cl 1,2-dichloroethane
Addition of Bromine:
CH2=CH2+Br2CH2Br-CH2Br 1,2-dibromoethane
Addition of H2O:
CH2=CH2+HOH → CH3-CH2-OH
sp2 Hybridization
The carbons in ethene (C2H4) have a double bond between them. Carbon will sp2 hybridise in this situation; sp2hybridization occurs when the 2s orbital mixes with just two of the three available 2p orbitals, resulting in three sp hybrid orbitals and one p-orbital. Each carbon forms three sigma bonds, which are explained by the three hybridised orbitals.
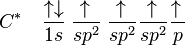
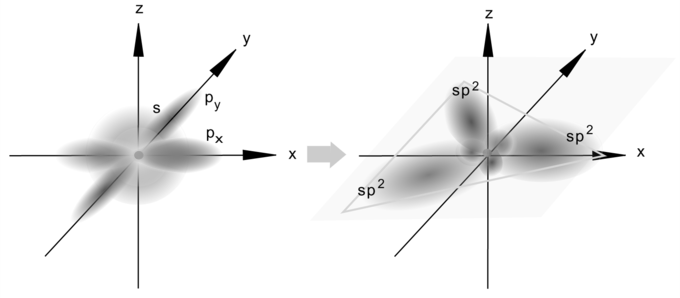
ethene sp2 hybridization. The 2s orbital mixes with just two of the three available 2p orbitals in sp2 hybridization, resulting in three sp2 orbitals and one p-orbital.
Four hydrogen atoms are joined to a pair of carbon atoms by a double bond in this hydrocarbon. The six atoms that make up ethylene are all parallel. The H-C-H angle is 117.4°, which is close to the ideal sp2 hybridised carbon angle of 120°. The molecule is also weak: rotation around the C-C bond is a low-energy process that requires breaking the -bond with heat at 50°C.
sp2 Hybrid
The two carbon atoms form a sigma bond in the molecule by overlapping two sp2orbitals. Each carbon atom forms two covalent bonds with hydrogen by s–sp2overlap, all with 120° angles. 2p–2p overlap forms the pi bond between carbon atoms perpendicular to the molecular plane.

Hybrid sp2orbitals are formed. An s-orbital combines with two p orbitals to generate three sp2hybrid orbitals, as seen in this diagram. Keep in mind that the three atomic orbitals produce the same number of hybrid orbitals.
Conclusion
Ethylene is used in two ways: as a monomer from which longer carbon chains can be built, and as a starting material for other two-carbon compounds. The first is the single greatest consumer of ethylene, accounting for over half of the annual output. Polymerization (the repeated merging of numerous small molecules into larger molecules) of ethylene produces polyethylene, a polymer with a wide range of applications, including packaging films, wire coatings, and squeeze bottles. Low-density polyethylene is generated when polymerization is carried out at high pressures and temperatures, and it has qualities that differ from high-density polyethylene formed under Ziegler-Natta catalytic conditions (see industrial polymers).
At 400°C, the aromatization of ethene over HZSM-5 zeolite was investigated at various space velocities and time-on-stream (TOS). As a result, experimental data were acquired for reactions carried out over either a fresh (TOS = 5 min) or a deactivated (TOS up to 4 hours) catalyst. The reaction experiments were backed up by an in situ EPR investigation of coke production. A thorough kinetic model for the ethane aromatization process on HZSM-5 zeolite under conditions of catalyst deactivation was established as a result of this work.